- Article
- Published: 11 September 2024
- Qianqian Lyu orcid.org/0009-0008-5070-29201,2 na1,
- Wenzhi Xue orcid.org/0000-0003-3453-17261,3 na1,
- Ruixin Liu orcid.org/0000-0002-5526-67171,2 na1,
- Qinyun Ma1,2 na1,
- Vikram Babu Kasaragod4 na1,
- Shan Sun5 na1,
- Qian Li1,
- Yanru Chen1,
- Mingyang Yuan orcid.org/0000-0003-2657-66761,
- Yuying Yang orcid.org/0000-0001-5167-32621,
- Bing Zhang orcid.org/0000-0001-8556-80495,
- Aifang Nie1,2,
- Sheng Jia1,
- Chongrong Shen1,
- Po Gao orcid.org/0000-0002-2860-32396,
- Weifang Rong6,
- Chenxi Yu7,
- Yufang Bi1,2,
- Chunlei Zhang orcid.org/0000-0003-1296-13708,
- Fajun Nan7,
- Guang Ning1,2,
- Zihe Rao5,
- Xiuna Yang orcid.org/0000-0003-3443-98155,
- Jiqiu Wang orcid.org/0000-0002-9383-76561,2 &
- …
- Weiqing Wang orcid.org/0000-0001-6027-30841,2
Nature (2024)Cite this article
- 1701 Accesses
- 119 Altmetric
Although fat is a crucial source of energy in diets, excessive intake leads to obesity. Fat absorption in the gut is prevailingly thought to occur organ-autonomously by diffusion1,2,3. Whether the process is controlled by the brain-to-gut axis, however, remains largely unknown. Here we demonstrate that the dorsal motor nucleus of vagus (DMV) plays a key part in this process. Inactivation of DMV neurons reduces intestinal fat absorption and consequently causes weight loss, whereas activation of the DMV increases fat absorption and weight gain. Notably, the inactivation of a subpopulation of DMV neurons that project to the jejunum shortens the length of microvilli, thereby reducing fat absorption. Moreover, we identify a natural compound, puerarin, that mimics the suppression of the DMV–vagus pathway, which in turn leads to reduced fat absorption. Photoaffinity chemical methods and cryogenic electron microscopy of the structure of a GABAA receptor–puerarin complex reveal that puerarin binds to an allosteric modulatory site. Notably, conditional Gabra1 knockout in the DMV largely abolishes puerarin-induced intestinal fat loss. In summary, we discover that suppression of the DMV–vagus–jejunum axis controls intestinal fat absorption by shortening the length of microvilli and illustrate the therapeutic potential of puerarin binding to GABRA1 in fat loss.
This is a preview of subscription content, access via your institution
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Fig. 1: The DMV controls jejunal fat absorption and body weight gain.

Fig. 2: Administration of puerarin suppresses DMV activity and leads to less jejunal fat absorption.

Fig. 3: Puerarin binds to the α1 subunit of the GABAA receptor to modulate DMV neurons.

Fig. 4: Jejunum-projecting DMV neurons control fat absorption and body weight gain.

Fig. 5: Suppression of the DMV–vagus pathway shortens jejunal microvilli to reduce fat absorption.

Article Open access 22 February 2024
Article Open access 07 September 2022

The MS data have been deposited into the ProteomeXchange Consortium through the PRIDE57 partner repository with the dataset identifier PXD052140. Atomic coordinates and the EM density map of the α1β3γ2L GABAA receptor bound to GABA and puerarin have been deposited into the PDB and Electron Microscopy Data Bank with the accession codes 9EQG and EMD-19907, respectively. The two structures used to compare pore diameters are from previously published reports29, with PDB accession codes 6HUP and 6HUK. Source data are provided with this paper.
- Barrett, K. E., Barman, S. M., Brooks, H. L. and Yuan, J. Ganong’s Review of Medical Physiology 26th edn. 475–476 (McGraw-Hill Medical, 2019).
- Hall, J. E. Guyton and Hall Textbook of Medical Physiology 13th edn., 835–841 (Elsevier, 2016).
- Koeppen, B. M. & Stanton, B. A. Berne & Levy Physiology 7th edn., 551–553 (Elsevier, 2018).
- Alberti, K. G. M. M., Zimmet, P. & Shaw, J. The metabolic syndrome—a new worldwide definition. Lancet 366, 1059–1062 (2005).
[Article](https://doi.org/10.1016%2FS0140-6736%2805%2967402-8) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=16182882) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=The%20metabolic%20syndrome%E2%80%94a%20new%20worldwide%20definition&journal=Lancet&doi=10.1016%2FS0140-6736%2805%2967402-8&volume=366&pages=1059-1062&publication_year=2005&author=Alberti%2CKGMM&author=Zimmet%2CP&author=Shaw%2CJ)
- Mourad, F. H. & Saade, N. E. Neural regulation of intestinal nutrient absorption. Prog. Neurobiol. 95, 149–162 (2011).
[Article](https://doi.org/10.1016%2Fj.pneurobio.2011.07.010) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BC3MXht1Gmsr7M) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=21854830) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Neural%20regulation%20of%20intestinal%20nutrient%20absorption&journal=Prog.%20Neurobiol.&doi=10.1016%2Fj.pneurobio.2011.07.010&volume=95&pages=149-162&publication_year=2011&author=Mourad%2CFH&author=Saade%2CNE)
- Hussain, M. M. Intestinal lipid absorption and lipoprotein formation. Curr. Opin. Lipidol. 25, 200–206 (2014).
[Article](https://doi.org/10.1097%2FMOL.0000000000000084) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BC2cXnsFeks7k%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=24751933) [PubMed Central](http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4265799) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Intestinal%20lipid%20absorption%20and%20lipoprotein%20formation&journal=Curr.%20Opin.%20Lipidol.&doi=10.1097%2FMOL.0000000000000084&volume=25&pages=200-206&publication_year=2014&author=Hussain%2CMM)
- Delacour, D., Salomon, J., Robine, S. & Louvard, D. Plasticity of the brush border—the yin and yang of intestinal homeostasis. Nat. Rev. Gastroenterol. Hepatol. 13, 161–174 (2016).
[Article](https://doi.org/10.1038%2Fnrgastro.2016.5) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BC28XitlCisr8%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=26837713) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Plasticity%20of%20the%20brush%20border%E2%80%94the%20yin%20and%20yang%20of%20intestinal%20homeostasis&journal=Nat.%20Rev.%20Gastroenterol.%20Hepatol.&doi=10.1038%2Fnrgastro.2016.5&volume=13&pages=161-174&publication_year=2016&author=Delacour%2CD&author=Salomon%2CJ&author=Robine%2CS&author=Louvard%2CD)
- Chivers, D. J. & Hladik, C. M. Morphology of the gastrointestinal tract in primates: comparisons with other mammals in relation to diet. J. Morphol. 166, 337–386 (1980).
[Article](https://doi.org/10.1002%2Fjmor.1051660306) [CAS](https://www.nature.com/articles/cas-redirect/1:STN:280:DyaL3M%2Fnt1ehsg%3D%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=7441763) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Morphology%20of%20the%20gastrointestinal%20tract%20in%20primates%3A%20comparisons%20with%20other%20mammals%20in%20relation%20to%20diet&journal=J.%20Morphol.&doi=10.1002%2Fjmor.1051660306&volume=166&pages=337-386&publication_year=1980&author=Chivers%2CDJ&author=Hladik%2CCM)
- Iqbal, J. et al. An intrinsic gut leptin–melanocortin pathway modulates intestinal microsomal triglyceride transfer protein and lipid absorption. J. Lipid Res. 51, 1929–1942 (2010).
[Article](https://doi.org/10.1194%2Fjlr.M005744) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=20164094) [PubMed Central](http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2882750) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=An%20intrinsic%20gut%20leptin%E2%80%93melanocortin%20pathway%20modulates%20intestinal%20microsomal%20triglyceride%20transfer%20protein%20and%20lipid%20absorption&journal=J.%20Lipid%20Res.&doi=10.1194%2Fjlr.M005744&volume=51&pages=1929-1942&publication_year=2010&author=Iqbal%2CJ)
- Travagli, R. A. & Anselmi, L. Vagal neurocircuitry and its influence on gastric motility. Nat. Rev. Gastroenterol. Hepatol. 13, 389–401 (2016).
[Article](https://doi.org/10.1038%2Fnrgastro.2016.76) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BC28XptFyisLg%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=27251213) [PubMed Central](http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5605144) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Vagal%20neurocircuitry%20and%20its%20influence%20on%20gastric%20motility&journal=Nat.%20Rev.%20Gastroenterol.%20Hepatol.&doi=10.1038%2Fnrgastro.2016.76&volume=13&pages=389-401&publication_year=2016&author=Travagli%2CRA&author=Anselmi%2CL)
- Browning, K. N. & Travagli, R. A. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr. Physiol. 4, 1339–1368 (2014).
[Article](https://doi.org/10.1002%2Fcphy.c130055) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=25428846) [PubMed Central](http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4858318) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Central%20nervous%20system%20control%20of%20gastrointestinal%20motility%20and%20secretion%20and%20modulation%20of%20gastrointestinal%20functions&journal=Compr.%20Physiol.&doi=10.1002%2Fcphy.c130055&volume=4&pages=1339-1368&publication_year=2014&author=Browning%2CKN&author=Travagli%2CRA)
- Browning, K. N. & Carson, K. E. Central neurocircuits regulating food intake in response to gut inputs—preclinical evidence. Nutrients 13, 908 (2021).
[Article](https://doi.org/10.3390%2Fnu13030908) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BB3MXhtFGisbrK) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=33799575) [PubMed Central](http://www.ncbi.nlm.nih.gov/pmc/articles/PMC7998662) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Central%20neurocircuits%20regulating%20food%20intake%20in%20response%20to%20gut%20inputs%E2%80%94preclinical%20evidence&journal=Nutrients&doi=10.3390%2Fnu13030908&volume=13&publication_year=2021&author=Browning%2CKN&author=Carson%2CKE)
- Doty, J. E. & Meyer, J. H. Vagotomy and antrectomy impairs canine fat absorption from solid but not liquid dietary sources. Gastroenterology 94, 50–56 (1988).
[Article](https://doi.org/10.1016%2F0016-5085%2888%2990608-7) [CAS](https://www.nature.com/articles/cas-redirect/1:STN:280:DyaL1c%2FosVanuw%3D%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=3335297) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Vagotomy%20and%20antrectomy%20impairs%20canine%20fat%20absorption%20from%20solid%20but%20not%20liquid%20dietary%20sources&journal=Gastroenterology&doi=10.1016%2F0016-5085%2888%2990608-7&volume=94&pages=50-56&publication_year=1988&author=Doty%2CJE&author=Meyer%2CJH)
- Zhang, Z., Lam, T. N. & Zuo, Z. Radix Puerariae: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharmacol. 53, 787–811 (2013).
[Article](https://doi.org/10.1002%2Fjcph.96) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=23677886) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Radix%20Puerariae%3A%20an%20overview%20of%20its%20chemistry%2C%20pharmacology%2C%20pharmacokinetics%2C%20and%20clinical%20use&journal=J.%20Clin.%20Pharmacol.&doi=10.1002%2Fjcph.96&volume=53&pages=787-811&publication_year=2013&author=Zhang%2CZ&author=Lam%2CTN&author=Zuo%2CZ)
- Urban, D. J. & Roth, B. L. DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annu. Rev. Pharmacol. Toxicol. 55, 399–417 (2015).
[Article](https://doi.org/10.1146%2Fannurev-pharmtox-010814-124803) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BC2MXkt12qs7g%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=25292433) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=DREADDs%20%28designer%20receptors%20exclusively%20activated%20by%20designer%20drugs%29%3A%20chemogenetic%20tools%20with%20therapeutic%20utility&journal=Annu.%20Rev.%20Pharmacol.%20Toxicol.&doi=10.1146%2Fannurev-pharmtox-010814-124803&volume=55&pages=399-417&publication_year=2015&author=Urban%2CDJ&author=Roth%2CBL)
- Mazzone, C. M. et al. Acute engagement of Gq-mediated signaling in the bed nucleus of the stria terminalis induces anxiety-like behavior. Mol. Psychiatry 23, 143–153 (2018).
[Article](https://doi.org/10.1038%2Fmp.2016.218) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BC28XitFeiurjI) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=27956747) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Acute%20engagement%20of%20Gq-mediated%20signaling%20in%20the%20bed%20nucleus%20of%20the%20stria%20terminalis%20induces%20anxiety-like%20behavior&journal=Mol.%20Psychiatry&doi=10.1038%2Fmp.2016.218&volume=23&pages=143-153&publication_year=2018&author=Mazzone%2CCM)
- Gautron, L., Zechner, J. F. & Aguirre, V. Vagal innervation patterns following Roux-en-Y gastric bypass in the mouse. Int. J. Obes. 37, 1603–1607 (2013).
[Article](https://doi.org/10.1038%2Fijo.2013.48) [CAS](https://www.nature.com/articles/cas-redirect/1:STN:280:DC%2BC3srnsV2gsA%3D%3D) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Vagal%20innervation%20patterns%20following%20Roux-en-Y%20gastric%20bypass%20in%20the%20mouse&journal=Int.%20J.%20Obes.&doi=10.1038%2Fijo.2013.48&volume=37&pages=1603-1607&publication_year=2013&author=Gautron%2CL&author=Zechner%2CJF&author=Aguirre%2CV)
- Borgstrom, B., Dahlqvist, A., Lundh, G. & Sjovall, J. Studies of intestinal digestion and absorption in the human. J. Clin. Invest. 36, 1521–1536 (1957).
[Article](https://doi.org/10.1172%2FJCI103549) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DyaG1cXns1ek) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=13475490) [PubMed Central](http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1072752) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Studies%20of%20intestinal%20digestion%20and%20absorption%20in%20the%20human&journal=J.%20Clin.%20Invest.&doi=10.1172%2FJCI103549&volume=36&pages=1521-1536&publication_year=1957&author=Borgstrom%2CB&author=Dahlqvist%2CA&author=Lundh%2CG&author=Sjovall%2CJ)
- Booth, C. C., Read, A. E. & Jones, E. Studies on the site of fat absorption: 1. The sites of absorption of increasing doses of I-labelled triolein in the rat. Gut 2, 23–31 (1961).
[Article](https://doi.org/10.1136%2Fgut.2.1.23) [CAS](https://www.nature.com/articles/cas-redirect/1:STN:280:DC%2BD1crjtlSguw%3D%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=18668739) [PubMed Central](http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1413205) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Studies%20on%20the%20site%20of%20fat%20absorption%3A%201.%20The%20sites%20of%20absorption%20of%20increasing%20doses%20of%20I-labelled%20triolein%20in%20the%20rat&journal=Gut&doi=10.1136%2Fgut.2.1.23&volume=2&pages=23-31&publication_year=1961&author=Booth%2CCC&author=Read%2CAE&author=Jones%2CE)
- Ludwig, M. Q. et al. A genetic map of the mouse dorsal vagal complex and its role in obesity. Nat. Metab. 3, 530–545 (2021).
[Article](https://doi.org/10.1038%2Fs42255-021-00363-1) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BB3MXhtlyru7rL) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=33767443) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=A%20genetic%20map%20of%20the%20mouse%20dorsal%20vagal%20complex%20and%20its%20role%20in%20obesity&journal=Nat.%20Metab.&doi=10.1038%2Fs42255-021-00363-1&volume=3&pages=530-545&publication_year=2021&author=Ludwig%2CMQ)
- Rossi, J. et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 13, 195–204 (2011).
[Article](https://doi.org/10.1016%2Fj.cmet.2011.01.010) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BC3MXhtlaisr0%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=21284986) [PubMed Central](http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3033043) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Melanocortin-4%20receptors%20expressed%20by%20cholinergic%20neurons%20regulate%20energy%20balance%20and%20glucose%20homeostasis&journal=Cell%20Metab.&doi=10.1016%2Fj.cmet.2011.01.010&volume=13&pages=195-204&publication_year=2011&author=Rossi%2CJ)
- Yang, M. et al. The effect of puerarin on carotid intima-media thickness in patients with active rheumatoid arthritis: a randomized controlled trial. Clin. Ther. 40, 1752–1764.e1 (2018).
[Article](https://doi.org/10.1016%2Fj.clinthera.2018.08.014) [ADS](http://adsabs.harvard.edu/cgi-bin/nph-data_query?link_type=ABSTRACT&bibcode=2018itp..book.....Y) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BC1cXhslalsrbI) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=30245282) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=The%20effect%20of%20puerarin%20on%20carotid%20intima-media%20thickness%20in%20patients%20with%20active%20rheumatoid%20arthritis%3A%20a%20randomized%20controlled%20trial&journal=Clin.%20Ther.&doi=10.1016%2Fj.clinthera.2018.08.014&volume=40&pages=1752-1764.e1&publication_year=2018&author=Yang%2CM)
- Niphakis, M. J. & Cravatt, B. F. Enzyme inhibitor discovery by activity-based protein profiling. Annu. Rev. Biochem. 83, 341–377 (2014).
[Article](https://doi.org/10.1146%2Fannurev-biochem-060713-035708) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BC2cXhtFOhtrjJ) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=24905785) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Enzyme%20inhibitor%20discovery%20by%20activity-based%20protein%20profiling&journal=Annu.%20Rev.%20Biochem.&doi=10.1146%2Fannurev-biochem-060713-035708&volume=83&pages=341-377&publication_year=2014&author=Niphakis%2CMJ&author=Cravatt%2CBF)
- Shieh, P., Siegrist, M. S., Cullen, A. J. & Bertozzi, C. R. Imaging bacterial peptidoglycan with near-infrared fluorogenic azide probes. Proc. Natl Acad. Sci. USA 111, 5456–5461 (2014).
[Article](https://doi.org/10.1073%2Fpnas.1322727111) [ADS](http://adsabs.harvard.edu/cgi-bin/nph-data_query?link_type=ABSTRACT&bibcode=2014PNAS..111.5456S) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BC2cXltl2lsbk%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=24706769) [PubMed Central](http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3992625) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Imaging%20bacterial%20peptidoglycan%20with%20near-infrared%20fluorogenic%20azide%20probes&journal=Proc.%20Natl%20Acad.%20Sci.%20USA&doi=10.1073%2Fpnas.1322727111&volume=111&pages=5456-5461&publication_year=2014&author=Shieh%2CP&author=Siegrist%2CMS&author=Cullen%2CAJ&author=Bertozzi%2CCR)
- Fritschy, J. M. & Mohler, H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J. Comp. Neurol. 359, 154–194 (1995).
[Article](https://doi.org/10.1002%2Fcne.903590111) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DyaK2MXotVagu70%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=8557845) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=GABAA-receptor%20heterogeneity%20in%20the%20adult%20rat%20brain%3A%20differential%20regional%20and%20cellular%20distribution%20of%20seven%20major%20subunits&journal=J.%20Comp.%20Neurol.&doi=10.1002%2Fcne.903590111&volume=359&pages=154-194&publication_year=1995&author=Fritschy%2CJM&author=Mohler%2CH)
- Sivarao, D. V., Krowicki, Z. K. & Hornby, P. J. Role of GABAA receptors in rat hindbrain nuclei controlling gastric motor function. Neurogastroenterol. Motil. 10, 305–313 (1998).
[Article](https://doi.org/10.1046%2Fj.1365-2982.1998.00110.x) [CAS](https://www.nature.com/articles/cas-redirect/1:STN:280:DyaK1czmt1Cgsw%3D%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=9697105) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Role%20of%20GABAA%20receptors%20in%20rat%20hindbrain%20nuclei%20controlling%20gastric%20motor%20function&journal=Neurogastroenterol.%20Motil.&doi=10.1046%2Fj.1365-2982.1998.00110.x&volume=10&pages=305-313&publication_year=1998&author=Sivarao%2CDV&author=Krowicki%2CZK&author=Hornby%2CPJ)
- Sigel, E. & Steinmann, M. E. Structure, function, and modulation of GABAA receptors. J. Biol. Chem. 287, 40224–40231 (2012).
[Article](https://doi.org/10.1074%2Fjbc.R112.386664) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BC38XhslansbjP) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=23038269) [PubMed Central](http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3504738) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Structure%2C%20function%2C%20and%20modulation%20of%20GABAA%20receptors&journal=J.%20Biol.%20Chem.&doi=10.1074%2Fjbc.R112.386664&volume=287&pages=40224-40231&publication_year=2012&author=Sigel%2CE&author=Steinmann%2CME)
- Laverty, D. et al. Cryo-EM structure of the human α1β3γ2 GABAA receptor in a lipid bilayer. Nature 565, 516–520 (2019).
[Article](https://doi.org/10.1038%2Fs41586-018-0833-4) [ADS](http://adsabs.harvard.edu/cgi-bin/nph-data_query?link_type=ABSTRACT&bibcode=2019Natur.565..516L) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BC1MXlslKmsL8%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=30602789) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Cryo-EM%20structure%20of%20the%20human%20%CE%B11%CE%B23%CE%B32%20GABAA%20receptor%20in%20a%20lipid%20bilayer&journal=Nature&doi=10.1038%2Fs41586-018-0833-4&volume=565&pages=516-520&publication_year=2019&author=Laverty%2CD)
- Masiulis, S. et al. GABAA receptor signalling mechanisms revealed by structural pharmacology. Nature 565, 454–459 (2019).
[Article](https://doi.org/10.1038%2Fs41586-018-0832-5) [ADS](http://adsabs.harvard.edu/cgi-bin/nph-data_query?link_type=ABSTRACT&bibcode=2019Natur.565..454M) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BC1MXlslKmsLw%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=30602790) [PubMed Central](http://www.ncbi.nlm.nih.gov/pmc/articles/PMC6370056) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=GABAA%20receptor%20signalling%20mechanisms%20revealed%20by%20structural%20pharmacology&journal=Nature&doi=10.1038%2Fs41586-018-0832-5&volume=565&pages=454-459&publication_year=2019&author=Masiulis%2CS)
- Zhu, S. et al. Structural and dynamic mechanisms of GABAA receptor modulators with opposing activities. Nat. Commun. 13, 4582 (2022).
[Article](https://doi.org/10.1038%2Fs41467-022-32212-4) [ADS](http://adsabs.harvard.edu/cgi-bin/nph-data_query?link_type=ABSTRACT&bibcode=2022NatCo..13.4582Z) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BB38XitFWlsrrF) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=35933426) [PubMed Central](http://www.ncbi.nlm.nih.gov/pmc/articles/PMC9357065) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Structural%20and%20dynamic%20mechanisms%20of%20GABAA%20receptor%20modulators%20with%20opposing%20activities&journal=Nat.%20Commun.&doi=10.1038%2Fs41467-022-32212-4&volume=13&publication_year=2022&author=Zhu%2CS)
- Kasaragod, V. B. et al. The molecular basis of drug selectivity for α5 subunit-containing GABAA receptors. Nat. Struct. Mol. Biol. 30, 1936–1946 (2023).
[Article](https://doi.org/10.1038%2Fs41594-023-01133-1) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BB3sXit1KgsrfJ) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=37903907) [PubMed Central](http://www.ncbi.nlm.nih.gov/pmc/articles/PMC10716045) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=The%20molecular%20basis%20of%20drug%20selectivity%20for%20%CE%B15%20subunit-containing%20GABAA%20receptors&journal=Nat.%20Struct.%20Mol.%20Biol.&doi=10.1038%2Fs41594-023-01133-1&volume=30&pages=1936-1946&publication_year=2023&author=Kasaragod%2CVB)
- Browning, K. N., Renehan, W. E. & Travagli, R. A. Electrophysiological and morphological heterogeneity of rat dorsal vagal neurones which project to specific areas of the gastrointestinal tract. J. Physiol. 517, 521–532 (1999).
[Article](https://doi.org/10.1111%2Fj.1469-7793.1999.0521t.x) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DyaK1MXktVyktro%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=10332099) [PubMed Central](http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2269359) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Electrophysiological%20and%20morphological%20heterogeneity%20of%20rat%20dorsal%20vagal%20neurones%20which%20project%20to%20specific%20areas%20of%20the%20gastrointestinal%20tract&journal=J.%20Physiol.&doi=10.1111%2Fj.1469-7793.1999.0521t.x&volume=517&pages=521-532&publication_year=1999&author=Browning%2CKN&author=Renehan%2CWE&author=Travagli%2CRA)
- Libbrecht, S., Van den Haute, C., Malinouskaya, L., Gijsbers, R. & Baekelandt, V. Evaluation of WGA-Cre-dependent topological transgene expression in the rodent brain. Brain Struct. Funct. 222, 717–733 (2017).
[Article](https://link.springer.com/doi/10.1007/s00429-016-1241-x) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BC28XpsVOntr8%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=27259586) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Evaluation%20of%20WGA-Cre-dependent%20topological%20transgene%20expression%20in%20the%20rodent%20brain&journal=Brain%20Struct.%20Funct.&doi=10.1007%2Fs00429-016-1241-x&volume=222&pages=717-733&publication_year=2017&author=Libbrecht%2CS&author=Haute%2CC&author=Malinouskaya%2CL&author=Gijsbers%2CR&author=Baekelandt%2CV)
- Bernard, C. Mémoire sur le Pancréas et sur le Role du Suc Pancréatique dans les Phénomènes Digestifs, Particulièrement dans la Digestion des Matières Grasses Neutres (J.-B. Baillière, 1856).
- Tao, J. et al. Highly selective brain-to-gut communication via genetically defined vagus neurons. Neuron 109, 2106–2115.e4 (2021).
[Article](https://doi.org/10.1016%2Fj.neuron.2021.05.004) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BB3MXhtlSrtrfI) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=34077742) [PubMed Central](http://www.ncbi.nlm.nih.gov/pmc/articles/PMC8273126) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Highly%20selective%20brain-to-gut%20communication%20via%20genetically%20defined%20vagus%20neurons&journal=Neuron&doi=10.1016%2Fj.neuron.2021.05.004&volume=109&pages=2106-2115.e4&publication_year=2021&author=Tao%2CJ)
- Tang, D. D. & Gunst, S. J. The small GTPase Cdc42 regulates actin polymerization and tension development during contractile stimulation of smooth muscle. J. Biol. Chem. 279, 51722–51728 (2004).
[Article](https://doi.org/10.1074%2Fjbc.M408351200) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BD2cXhtVCktb3E) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=15456777) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=The%20small%20GTPase%20Cdc42%20regulates%20actin%20polymerization%20and%20tension%20development%20during%20contractile%20stimulation%20of%20smooth%20muscle&journal=J.%20Biol.%20Chem.&doi=10.1074%2Fjbc.M408351200&volume=279&pages=51722-51728&publication_year=2004&author=Tang%2CDD&author=Gunst%2CSJ)
- Berryman, M., Franck, Z. & Bretscher, A. Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J. Cell Sci. 105, 1025–1043 (1993).
[Article](https://doi.org/10.1242%2Fjcs.105.4.1025) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DyaK2cXhsF2msb4%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=8227193) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Ezrin%20is%20concentrated%20in%20the%20apical%20microvilli%20of%20a%20wide%20variety%20of%20epithelial%20cells%20whereas%20moesin%20is%20found%20primarily%20in%20endothelial%20cells&journal=J.%20Cell%20Sci.&doi=10.1242%2Fjcs.105.4.1025&volume=105&pages=1025-1043&publication_year=1993&author=Berryman%2CM&author=Franck%2CZ&author=Bretscher%2CA)
- Travagli, R. A., Gillis, R. A., Rossiter, C. D. & Vicini, S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am. J. Physiol. 260, G531–G536 (1991).
[CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DyaK3MXhslyrs7Y%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=1672243) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Glutamate%20and%20GABA-mediated%20synaptic%20currents%20in%20neurons%20of%20the%20rat%20dorsal%20motor%20nucleus%20of%20the%20vagus&journal=Am.%20J.%20Physiol.&volume=260&pages=G531-G536&publication_year=1991&author=Travagli%2CRA&author=Gillis%2CRA&author=Rossiter%2CCD&author=Vicini%2CS)
- Guerciolini, R. Mode of action of orlistat. Int. J. Obes. Relat. Metab. Disord. 21, S12–S23 (1997).
[CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DyaK2sXlt1Smu7w%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=9225172) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Mode%20of%20action%20of%20orlistat&journal=Int.%20J.%20Obes.%20Relat.%20Metab.%20Disord.&volume=21&pages=S12-S23&publication_year=1997&author=Guerciolini%2CR)
- Douglas, I. J., Langham, J., Bhaskaran, K., Brauer, R. & Smeeth, L. Orlistat and the risk of acute liver injury: self controlled case series study in UK Clinical Practice Research Datalink. BMJ 346, f1936 (2013).
[Article](https://doi.org/10.1136%2Fbmj.f1936) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=23585064) [PubMed Central](http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3624963) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Orlistat%20and%20the%20risk%20of%20acute%20liver%20injury%3A%20self%20controlled%20case%20series%20study%20in%20UK%20Clinical%20Practice%20Research%20Datalink&journal=BMJ&doi=10.1136%2Fbmj.f1936&volume=346&publication_year=2013&author=Douglas%2CIJ&author=Langham%2CJ&author=Bhaskaran%2CK&author=Brauer%2CR&author=Smeeth%2CL)
- Capasso, R. et al. Fatty acid amide hydrolase controls mouse intestinal motility in vivo. Gastroenterology 129, 941–951 (2005).
[Article](https://doi.org/10.1053%2Fj.gastro.2005.06.018) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BD2MXhtV2isrjL) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=16143133) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Fatty%20acid%20amide%20hydrolase%20controls%20mouse%20intestinal%20motility%20in%20vivo&journal=Gastroenterology&doi=10.1053%2Fj.gastro.2005.06.018&volume=129&pages=941-951&publication_year=2005&author=Capasso%2CR)
- Toda, C. et al. UCP2 regulates mitochondrial fission and ventromedial nucleus control of glucose responsiveness. Cell 164, 872–883 (2016).
[Article](https://doi.org/10.1016%2Fj.cell.2016.02.010) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BC28XjsValsbc%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=26919426) [PubMed Central](http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4770556) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=UCP2%20regulates%20mitochondrial%20fission%20and%20ventromedial%20nucleus%20control%20of%20glucose%20responsiveness&journal=Cell&doi=10.1016%2Fj.cell.2016.02.010&volume=164&pages=872-883&publication_year=2016&author=Toda%2CC)
- Jackson, P. & Lapinsky, D. J. Appendage and scaffold diverse fully functionalized small-molecule probes via a minimalist terminal alkyne-aliphatic diazirine isocyanide. J. Org. Chem. 83, 11245–11253 (2018).
[Article](https://doi.org/10.1021%2Facs.joc.8b01831) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BC1cXhsFGmsbfP) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=30132667) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Appendage%20and%20scaffold%20diverse%20fully%20functionalized%20small-molecule%20probes%20via%20a%20minimalist%20terminal%20alkyne-aliphatic%20diazirine%20isocyanide&journal=J.%20Org.%20Chem.&doi=10.1021%2Facs.joc.8b01831&volume=83&pages=11245-11253&publication_year=2018&author=Jackson%2CP&author=Lapinsky%2CDJ)
- Gao, J., Mfuh, A., Amako, Y. & Woo, C. M. Small molecule interactome mapping by photoaffinity labeling reveals binding site hotspots for the NSAIDs. J. Am. Chem. Soc. 140, 4259–4268 (2018).
[Article](https://doi.org/10.1021%2Fjacs.7b11639) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BC1cXksFemu7Y%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=29543447) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Small%20molecule%20interactome%20mapping%20by%20photoaffinity%20labeling%20reveals%20binding%20site%20hotspots%20for%20the%20NSAIDs&journal=J.%20Am.%20Chem.%20Soc.&doi=10.1021%2Fjacs.7b11639&volume=140&pages=4259-4268&publication_year=2018&author=Gao%2CJ&author=Mfuh%2CA&author=Amako%2CY&author=Woo%2CCM)
- Dostalova, Z. et al. Human α1β3γ2L γ-aminobutyric acid type A receptors: high-level production and purification in a functional state. Protein Sci. 23, 157–166 (2014).
[Article](https://doi.org/10.1002%2Fpro.2401) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BC2cXps1Wisg%3D%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=24288268) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Human%20%CE%B11%CE%B23%CE%B32L%20%CE%B3-aminobutyric%20acid%20type%20A%20receptors%3A%20high-level%20production%20and%20purification%20in%20a%20functional%20state&journal=Protein%20Sci.&doi=10.1002%2Fpro.2401&volume=23&pages=157-166&publication_year=2014&author=Dostalova%2CZ)
- Kimanius, D., Dong, L., Sharov, G., Nakane, T. & Scheres, S. H. W. New tools for automated cryo-EM single-particle analysis in RELION-4.0. Biochem. J. 478, 4169–4185 (2021).
[Article](https://doi.org/10.1042%2FBCJ20210708) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BB38XhsVaru7k%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=34783343) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=New%20tools%20for%20automated%20cryo-EM%20single-particle%20analysis%20in%20RELION-4.0&journal=Biochem.%20J.&doi=10.1042%2FBCJ20210708&volume=478&pages=4169-4185&publication_year=2021&author=Kimanius%2CD&author=Dong%2CL&author=Sharov%2CG&author=Nakane%2CT&author=Scheres%2CSHW)
- Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
[Article](https://doi.org/10.1038%2Fnmeth.4193) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BC2sXjt1ags7g%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=28250466) [PubMed Central](http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5494038) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=MotionCor2%3A%20anisotropic%20correction%20of%20beam-induced%20motion%20for%20improved%20cryo-electron%20microscopy&journal=Nat.%20Methods&doi=10.1038%2Fnmeth.4193&volume=14&pages=331-332&publication_year=2017&author=Zheng%2CSQ)
- Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
[Article](https://doi.org/10.1016%2Fj.jsb.2015.08.008) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=26278980) [PubMed Central](http://www.ncbi.nlm.nih.gov/pmc/articles/PMC6760662) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=CTFFIND4%3A%20fast%20and%20accurate%20defocus%20estimation%20from%20electron%20micrographs&journal=J.%20Struct.%20Biol.&doi=10.1016%2Fj.jsb.2015.08.008&volume=192&pages=216-221&publication_year=2015&author=Rohou%2CA&author=Grigorieff%2CN)
- Tegunov, D. & Cramer, P. Real-time cryo-electron microscopy data preprocessing with Warp. Nat. Methods 16, 1146–1152 (2019).
[Article](https://doi.org/10.1038%2Fs41592-019-0580-y) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BC1MXhvFejs77E) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=31591575) [PubMed Central](http://www.ncbi.nlm.nih.gov/pmc/articles/PMC6858868) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Real-time%20cryo-electron%20microscopy%20data%20preprocessing%20with%20Warp&journal=Nat.%20Methods&doi=10.1038%2Fs41592-019-0580-y&volume=16&pages=1146-1152&publication_year=2019&author=Tegunov%2CD&author=Cramer%2CP)
- Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
[Article](https://doi.org/10.1038%2Fnmeth.4169) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BC2sXitlGisbs%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=28165473) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=cryoSPARC%3A%20algorithms%20for%20rapid%20unsupervised%20cryo-EM%20structure%20determination&journal=Nat.%20Methods&doi=10.1038%2Fnmeth.4169&volume=14&pages=290-296&publication_year=2017&author=Punjani%2CA&author=Rubinstein%2CJL&author=Fleet%2CDJ&author=Brubaker%2CM)
- Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D Struct. Biol. 75, 861–877 (2019).
[Article](https://doi.org/10.1107%2FS2059798319011471) [ADS](http://adsabs.harvard.edu/cgi-bin/nph-data_query?link_type=ABSTRACT&bibcode=2019AcCrD..75..861L) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BC1MXhvFWkurrO) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=31588918) [PubMed Central](http://www.ncbi.nlm.nih.gov/pmc/articles/PMC6778852) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Macromolecular%20structure%20determination%20using%20X-rays%2C%20neutrons%20and%20electrons%3A%20recent%20developments%20in%20Phenix&journal=Acta%20Crystallogr.%20D%20Struct.%20Biol.&doi=10.1107%2FS2059798319011471&volume=75&pages=861-877&publication_year=2019&author=Liebschner%2CD)
- Casanal, A., Lohkamp, B. & Emsley, P. Current developments in Coot for macromolecular model building of electron cryo-microscopy and crystallographic data. Protein Sci. 29, 1069–1078 (2020).
[Article](https://doi.org/10.1002%2Fpro.3791) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BB3cXjvFyrtrw%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=31730249) [PubMed Central](http://www.ncbi.nlm.nih.gov/pmc/articles/PMC7096722) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Current%20developments%20in%20Coot%20for%20macromolecular%20model%20building%20of%20electron%20cryo-microscopy%20and%20crystallographic%20data&journal=Protein%20Sci.&doi=10.1002%2Fpro.3791&volume=29&pages=1069-1078&publication_year=2020&author=Casanal%2CA&author=Lohkamp%2CB&author=Emsley%2CP)
- Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
[Article](https://doi.org/10.1107%2FS0907444909042073) [ADS](http://adsabs.harvard.edu/cgi-bin/nph-data_query?link_type=ABSTRACT&bibcode=2010AcCrD..66...12C) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BC3cXit1Kktg%3D%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=20057044) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=MolProbity%3A%20all-atom%20structure%20validation%20for%20macromolecular%20crystallography&journal=Acta%20Crystallogr.%20D%20Biol.%20Crystallogr.&doi=10.1107%2FS0907444909042073&volume=66&pages=12-21&publication_year=2010&author=Chen%2CVB)
- Smart, O. S., Neduvelil, J. G., Wang, X., Wallace, B. A. & Sansom, M. S. HOLE: a program for the analysis of the pore dimensions of ion channel structural models. J. Mol. Graph. 14, 354–360, 376 (1996).
[Article](https://doi.org/10.1016%2FS0263-7855%2897%2900009-X) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DyaK2sXjvFGlsro%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=9195488) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=HOLE%3A%20a%20program%20for%20the%20analysis%20of%20the%20pore%20dimensions%20of%20ion%20channel%20structural%20models&journal=J.%20Mol.%20Graph.&doi=10.1016%2FS0263-7855%2897%2900009-X&volume=14&pages=354-360%2C%20376&publication_year=1996&author=Smart%2COS&author=Neduvelil%2CJG&author=Wang%2CX&author=Wallace%2CBA&author=Sansom%2CMS)
- Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007).
[Article](https://doi.org/10.1016%2Fj.jmb.2007.05.022) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BD2sXpvFGktb8%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=17681537) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=Inference%20of%20macromolecular%20assemblies%20from%20crystalline%20state&journal=J.%20Mol.%20Biol.&doi=10.1016%2Fj.jmb.2007.05.022&volume=372&pages=774-797&publication_year=2007&author=Krissinel%2CE&author=Henrick%2CK)
- Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
[Article](https://doi.org/10.1002%2Fpro.3943) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BB3cXitFamtrzJ) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=32881101) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=UCSF%20ChimeraX%3A%20structure%20visualization%20for%20researchers%2C%20educators%2C%20and%20developers&journal=Protein%20Sci.&doi=10.1002%2Fpro.3943&volume=30&pages=70-82&publication_year=2021&author=Pettersen%2CEF)
- Perez-Riverol, Y. et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 50, D543–D552 (2022).
[Article](https://doi.org/10.1093%2Fnar%2Fgkab1038) [CAS](https://www.nature.com/articles/cas-redirect/1:CAS:528:DC%2BB38Xis1ChtLo%3D) [PubMed](http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=34723319) [Google Scholar](http://scholar.google.com/scholar_lookup?&title=The%20PRIDE%20database%20resources%20in%202022%3A%20a%20hub%20for%20mass%20spectrometry-based%20proteomics%20evidences&journal=Nucleic%20Acids%20Res.&doi=10.1093%2Fnar%2Fgkab1038&volume=50&pages=D543-D552&publication_year=2022&author=Perez-Riverol%2CY)
We thank C.-C. Hui, Y. A. Zeng, W. Shen, J. Hu, C. Zhan and X. R. Ma for their suggestions on this study; G. Shu for the gift of Rosa26-tdTomato mice and N. Xu for Chat-cre mice; Y. Chen for confocal technical support; R. Piskorowski for providing puerarin for cryo-EM analysis; J. Grimmett, T. Darling and I. Clayson for scientific computing support; S. Chen, G. Cannone, G. Sharov, A. Yeates and B. Ahsan for EM support; staff at the Core Facility of Basic Medical Sciences, SJTUSM for EM technical support; and A. R. Aricescu for providing manuscript comments, materials and access to cryo-EM infrastructure. This work was supported by grants from the National Natural Science Foundation of China (82088102 and 91957124), the National Key Research and Development Program of China (2022YFC2505201 and 2021YFA1301103), the National Natural Science Foundation of China (92157204, 82250901, 81930021, 91857205 and 82100905), the Innovative research team of high-level local universities in Shanghai, Shanghai Municipal Education Commission (2023ZKZD22), the Science and Technology Commission of Shanghai Municipality (21JC1404400), and the Shanghai Sailing Program (21YF1440700), NATCM’s Project of High-level Construction of Key TCM Disciplines (ZYYZDXK-2023070). The cryo-EM work was funded by an EMBO long-term fellowship (ALTF137-2019), a Marie Sklodowska Curie individual fellowship (GABAARComp-897707) and the UK Medical Research Council (MC_UP_1201/15).
The authors declare no competing interests.
Nature thanks A. Radu Aricescu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
a-c, Representative whole cell patch-clamp recordings from a DMV neuron expressing the hM4D(Gi), before (black dashed box), during (red dashed box) and after CNO application (grey dashed box, wash) (a). Summary of average action potential (AP) frequency (b), and mean membrane potential (c) of a total of 14 neurons. d-f, Representative whole cell patch-clamp recordings from a DMV neuron expressing the hM3D(Gq), before (black dashed box), during (blue dashed box) and after CNO application (grey dashed box, wash) (d). Summary of average AP frequency (e), and mean membrane potential (f) of a total of 15 neurons. Data were analyzed using paired one-way ANOVA with Holm-Šídák’s multiple comparisons test. Data are presented as mean ± S.E.M. * P < 0.05; ** P < 0.01; *** P < 0.001; NS, no significance. Detailed statistics see source data.
a, Schematic of chemogenetic activation and inactivation of DMV neurons in mice fed on HFD. b-h, CNO-induced vagal neuronal manipulation in DMV by DREADDS system in activation (3q, n = 9), suppression (4i, n = 10) and control (con, n = 10) mice. Body weight before CNO application (b) and body weight change (c) during 20 days, 24 h fecal NEFA (d) and TG contents (e), jejunal (f), duodenal (g) and ileal (h) TG contents at day 20. Samples were collected 2 h after 200 μL olive oil gavage (f to h). Data were analyzed using two-way ANOVA (c) and one-way ANOVA with Holm-Šídák’s multiple comparisons test (b, d to h). Data are presented as mean ± S.E.M. ns, no significance; * P < 0.05; ** P < 0.01; *** P < 0.001. Detailed statistics see source data.
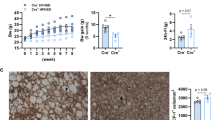
a, Schematic of the subdiaphragmatic vagotomy and sham mice fed on HFD (n = 6 per group). b, Body weight change of two groups. c, 24 h fecal NEFA contents. d, 24 h fecal TG contents. e, Jejunal TG contents of two groups with tissue collection 2 h after 200 μL olive oil gavage. Data were analyzed using two-way ANOVA (b), two-tailed Student’s t test (c and e) and two-tailed Mann-Whitney test (d). Data are presented as mean ± S.E.M. * P < 0.05, ** P < 0.01, *** P < 0.001. Detailed statistics see source data.
a, Chemical structure of puerarin molecule. b-j, cFOS immunostaining of brain regions in mice treated with i.p. injection of puerarin and vehicle under pair-feeding HFD, and counts of c-fos positive neurons in various rostral-caudal nucleus (n = 4 per group), including striatum (CPu) and bed nucleus of stria terminalis (BST) (b), paraventricular hypothalamus (PVH) (c), dorsomedial hypothalamus (DMH) (d), ventromedial hypothalamus (VMH) and arcuate nucleus of hypothalamus (ARC) (e), lateral hypothalamus (LH) (f), basolateral and central amygdaloid nucleus (BLA and CeA, Amygdala) (g), ventral tegmental area (VTA) and substantia nigra (SN) (h), dorsomedial, dorsolateral, lateral and ventrolateral periaqueductal gray (PAG including DMPAG, DLPAG, LPAG, and VLPAG) (i), and parabrachial nucleus (PBN) (j). Scale bar, 500 μm (b, h), 200 μm (d-g, i-j) and 100 μm (c). Data were analyzed using two-tailed Student’s t test and are presented as mean ± S.E.M. Veh, vehicle; Pue, puerarin. ns, no significance. Detailed statistics see source data.
a, Simplified chemical reaction of puerarin-tag synthesis. b-f, Body weight before injection (b), body weight change (c), 24 h fecal NEFA (d) and TG (e) contents, and jejunal TG contents (f) in mice treated with puerarin-tag and vehicle i.p. injection, respectively (n = 6 per group). g, Representative images of GABRA1 immunostaining in striatum (CPu), bed nucleus of stria terminalis (BST), paraventricular hypothalamus (PVH), dorsomedial hypothalamus (DMH), ventromedial hypothalamus (VMH), arcuate nucleus of hypothalamus (ARC), lateral hypothalamus (LH), basolateral and central amygdaloid nucleus (BLA and CeA, Amygdala), ventral tegmental area (VTA), substantia nigra (SN), dorsomedial, dorsolateral, lateral and ventrolateral periaqueductal gray (PAG, including DMPAG, DLPAG, LPAG, and VLPAG), parabrachial nucleus (PBN), nucleus of solitary tract (NTS), area postrema (AP), and dorsal motor nucleus of vagus (DMV). Scale bar, 100 μm. Data were analyzed using two-way ANOVA (c) and two-tailed Student’s t test (b, d to f). Data are presented as mean ± S.E.M. Veh, vehicle; Pue, puerarin. * P < 0.05, **P < 0.01, *** P < 0.001. Detailed statistics see source data.
a, Representative whole-cell current traces elicited from human α1β3γ2L stably expressed HEK293S cell line by puerarin alone. b, Fourier shell correlation (FSC) curves of the cryo-EM density map reconstruction (phase-randomized in red, masked in blue) and model vs. map correlation (orange) of the α1β3γ2L GABAA receptor bound to puerarin. c, The final unsharpened map coloured according to the local resolution estimated by RELION. d, Cryo-EM density map of the GABAA receptor-puerarin complex. The receptor subunits are coloured in red (α1), blue (β3) and yellow (γ2). The puerarin density, observed only at the α1+/γ2− interface, is coloured in cyan. The dashed rectangle marks the puerarin binding pocket. e-f, Enlarged views of the binding pocket where the density map is semi-transparent (light blue), the protein main chains are in cartoon representation, puerarin and the neighbouring side chains are in stick representation. g, Cartoon representation of the GABAA receptor bound to puerarin. Lipids surrounding the transmembrane region are in the ball and stick representation, while GABA and puerarin are shown as spheres. h-i, Top view cartoon representations of the puerarin-bound GABAA receptor transmembrane domain (TMD, coloured according to panel g) superimposed with structures of the bicuculline-bound α1β3γ2L (h, PDB:6HUK) and diazepam-bound α1β3γ2L (i, PDB:6HUP). The side chains of the 9′-activation gate leucine residues are shown in ball and stick representation. j, Channel pore radii profiles of the GABAA receptor bound to puerarin (cyan), bicuculline (black, PDB:6HUK) and diazepam (red, PDB:6HUP).
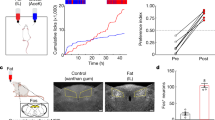
a, Construction strategy of Gabra1 vagal conditional knockout mice. b, Representative GABRA1 fluorescent staining and quantification in DMV of cKO and flox control mice (n = 4 per group). c, Representative whole cell patch-clamp recordings from a DMV neuron of control mouse (left), and a DMV neuron from cKO mouse (right). d-e, Average AP frequency (d), and mean membrane potential (e) of recorded DMV neurons from flox (n = 29) and cKO mice (n = 39). f, AP frequency and percentage of DMV neurons from flox (n = 20) and cKO (n = 21) mice showing puerarin-associated inhibition or non-inhibition. g, The body weight of flox and cKO mice before puerarin and vehicle injection (n = 8 per group). h, The body weight of WGA-con and WGA-4i before CNO application (n = 10 per group). Scale bar, 100 μm (b). Data were analyzed using two-tailed Student’s t test (b and h) and two-tailed Mann-Whitney test (d to e), two-sided Chi-square test (f), one-way ANOVA with Holm-Šídák’s multiple comparisons test (f) and two-way ANOVA with Tukey’s multiple comparisons test (g). Data are presented as mean ± S.E.M. * P < 0.05; ** P < 0.01; *** P < 0.001. Detailed statistics see source data.
a, The expression of genes related to jejunal lipid transporters in Px2b-4i and Px2b-con mice. b-d, HE staining of the jejunum (b), morphometric measurement of length distribution (c) and average length of jejunal villi (d) in Px2b-4i and Px2b-con mice (n = 4/group). e-g, HE staining of the jejunum (e), morphometric measurement of length distribution (f) and average length of jejunal villi (g) in puerarin- and vehicle-injected mice (n = 4 per group). h-j, Representative electron micrographs (h), morphometric measurement of length distribution (i) and average microvillus length (j) in the jejunum of 3q, 4i and con mice (n = 4 per group). k, The expression of genes related to microvillus length in jejunum of the three groups. l-n, Representative electron micrographs (l), morphometric measurement of length distribution (m) and average microvillus length (n) in the jejunum of puerarin- and vehicle-treated mice (n = 4 per group). o, The expression of genes related to microvillus length in jejunum of the two groups. Scale bar, 100 μm for optical micrographs; 1 μm for electron micrographs. Data were analyzed using two-tailed Student’s t test and two-tailed Mann-Whitney test (a to g, and m to o) and one-way ANOVA with Holm-Šídák’s multiple comparisons test (i to k). Data are presented as mean ± S.E.M. Veh, vehicle; Pue, puerarin; Px2b, Phox2b. * indicates Px2b-con vs. Px2b-4i or Veh vs. Pue or con vs. 4i, # Indicates con vs. 3q. *, # P < 0.05; **, ## P < 0.01; ***, ### P < 0.001. Detailed statistics see source data.
a-d, Representative electron micrographs (a), morphometric measurement of length distribution (b), average microvillus length (c) and related gene expression (d) in the duodenum of 3q, 4i and con mice. e-h, Representative electron micrographs (e), morphometric measurement of length distribution (f), average microvillus length (g), and related gene expression (h) in the ileum of the three groups. i-l, Representative electron micrographs (i), morphometric measurement of length distribution (j), average microvillus length (k) and related gene expression (l) in the duodenum of Puerarin- and vehicle-injected mice. m-p, Representative electron micrographs (m), morphometric measurement of length distribution (n), average microvillus length (o) and related gene expression (p) in the ileum of the two groups. Scale bar, 1 μm for electron micrographs. For electron microscopy analysis, n = 4 mice in each group (b, c, f, g, j, k, n and o). Data were analyzed using one-way ANOVA with Holm-Šídák’s multiple comparisons test (a to h), two-tailed Student’s t test and two-tailed Mann-Whitney test (i to p). Data are presented as mean ± S.E.M. Veh, vehicle; Pue, puerarin; Px2b, Phox2b. Detailed statistics see source data source data.
a, Schematic of a brain-to-gut axis controlling fat absorption in the jejunum, in which inhibition of DMV by chemogenetics or Puerarin leads to microvilli shortening, fat absorption suppression and consequently weight loss. (Figure was created with BioRender).
Extended Data Table 1 Cryo-EM data collection, atomic model refinement and validation statistics
Proteins found only in the puerarin-tag group by LC–MS.
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.